Pearls
have been known to mankind since the beginning of civilization. These structures are secreted by the mantle (i.e., the skin) of pearl oysters
in response to irritations caused by external or internal stimuli such as sand
grains, molluscs
eggs, parasites, detritus, and other foreign particles. India has one of the highest demand for pearls for setting in jewellry, and
is particularly famous for its pearl oyster resources which yield superb
pearls. The
pearl oyster fisheries are located in two main areas: 1) in the Gulf of Mannar off Tuticorin
coast and 2) in the Gulf of Kutch on the northwest coast of the country. Pinctada fucata,
pinctada
vulgarisare the
two important species & are commetrcially
important source of “ornamental pearls”.
BIOLOGY OF PEARL OYSTER
1. Food and feeding habits
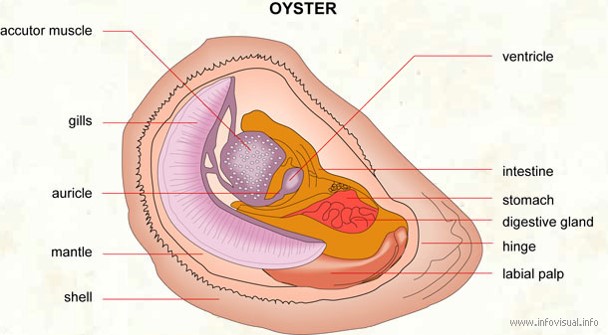
Like other bivalves, the pearl oyster is a filter feeder.Unicellular organisms including infusorians, foraminifers and radiolarians have been found in the stomach of pearl oyster. Minute embryos and larvae of various organisms, algal filaments, spicules of alcyonarians and sponges were also observed.
2. Reproduction
In pearl oysters, the sexes are separate although hermaphrodite conditions have been observed in some individuals. Change of sex takes place in some oyster towards the end of spawning.
Based on the external appearance, microscopic examination of smears and histological studies, five developmental stages have been distinguished in the gonads of P. fucata off Tuticorin coast.
Stage 1: Inactive/spent/resting
The gonad is completely shrunken and translucent. In some cases it is pale orange in colour. Large vacuolated yellow (fat) cells are seen in the interfollicular spaces. The sex at this stage can hardly be distinguished.
Stage 2: Developing/maturing
The transparent nature of the resting gonad is lost and it becomes distinguished from other visceral masses. Gametogenic materials begin to appear in the gonad. As the stage advances, the gonad begins to branch along the posterior side of the retractor muscle and advances to the anterio-dorsal region. The gametes begin to proliferate along the follicle wall. In advanced stages, the inter-follicular spaces become reduced and the lumen of the follicle may contain some free oocytes. The majority of the oocytes are irregular in shape and the germinal vesicle (nucleus) is not distinctly seen. The average size of the oocytes is 60.0 × 47.5 μm and the germinal vesicle, if present, is 20.0 μm.
Stage 3: Mature
The gonad spreads on to most of the visceral tissues. It is mostly yellowish cream. The lumen of the follicle is filled with free oocytes. Some of them are attached to the follicular wall by means of slender stalks. The majority of the oocytes are pyriform in shape. The average size of the oocyte is 68.0 x 50 μm with a well defined germinal vesicle. The mean diameter of the nucleus is 25 μm.
Stage 4: Partially spawned
The gonads become loose in consistency and the visceral epithelium becomes dull. The follicles shrink with the reduction of gametes in the lumen. The oocytes are free and found along the follicular wall. Most of the oocytes are spherical and nucleated. The average size of the oocyte is 51.7 μm.
Stage 5: Spent
The gonads shrink further with a few left over gametes in the lumen of the follicles. Ruptured follicles are seen in some cases and the lumen sometimes contains ruptured cells. Oocytes, if present are few and spherical. The average size of the oocytes is 54.4 μm. The description of the spent stages applies to the oysters which have recently undergone oogenesis. Otherwise they transform to the spent resting stage quickly.Males show the same pattern of reproductive activity. However, in stages 2 and 3, the colour of the gonad is pale cream. In other stages of gametogenesis, the gonads of males and females appear similar when observed externally.
HATCHERY TECHNIQUES FOR SEED PRODUCTION
Artificially reared spat
Seed of P. fucata, were produced in 1981 in the laboratory through hatchery techniques at the Central Marine Fisheries Research Institute at Tuticorin.
Hatchery building
The roof of the hatchery building is sufficiently high to avoid high temperature.
Part of the roof of the wet laboratory has translucent fibreglass sheets to allow sufficient light for indoor phytoplankton culture.Glass panelled large windows and ventilators are provided for free passage of light and air. The concrete floor has sufficient gradient facilities for easy drainage.
Seawater management
The seawater is usually drawn from the sea beyond the low water mark into a well through PVC pipes. The seawater is pumped to sedimentation tanks and passed onto the biological filter which contains coarse river sand at the top, pebbles below it and charcoal at the bottom. The filtered seawater is stored in a water sump and lifted to an overhead tank for supply to the hatchery. Periodic cleaning of the filter bed keeps the seawater uncontaminated. PVC, fibreglass and stainless steel materials are used in the hatchery. The seawater sterilized by ultraviolet irradiation is used only in specific cases.
Aeration
Air compressors with storage tank are used to aerate seawater in the rearing tanks. The compressed air is passed through a series of filters to remove oil and moisture and is supplied to the various culture vessels through PVC pipes. The air is supplied to the tanks through diffuser stones.
Live food production- Phytoplankton
Flagellates measuring less than 10 μm form the main food for pearl oyster larvae. Isochrysis galbana is an important algal food for the larvae. Other microalgal cells such as Pavlova, Chromulina and Dicrateria are also suitable for the larvae.
Broodstock maintenance
Oyster broodstock are maintained at a water temperature ranging from 25–28 °C in a controlled room. They are fed with a mixed algal diet at a ration of 4 l per oyster/day. The algal food is supplemented by raw corn flour at 30 mg per oyster/day. Pearl oysters with maturing gonad fed with the above food for 45 days will spawn with a 30 % response. The matured oysters can be kept for a prolonged period at 25–28 °C, while spawning of these oysters can be stimulated by raising the water temperature by few degrees.
Spawning
Spawning of natural oysters with mature gonads occurs when there is a simple change in the seawater environment or a mechanical shock by shell cleaning or a change in water pressure. In all cases males spawn first and this induces the females to spawn within 30 minutes.
In the absence of natural spawning the technique of induce spawning is employed. In this technique thermal stimulation is adopted predominantly by gradually increasing the water temperature by several degrees (from 28.5 °C to 35.0 °C).Spawning of pearl oysters can also be effected by chemical stimulation. Different concentrations (1.532, 3.064 and 6.128 millimolars) of hydrogen peroxide in combination either with normal seawater or alkaline seawater (pH 9.1) is used in inducing spawning. Different pH media (8.5, 9.0, 9.5 and 10.0) are prepared either using Tris buffer or Sodium hydroxide pellets (NaOH) and the pearl oysters are induced to spawn.
Fertilization
When the eggs are released in the medium, they are pyriform in shape measuring 73.9 μm along the long axis and 45.2 μm in width. The yolk cytoplasm is heavily granulated and opaque. The egg is enclosed in a vitelline membrane and a large germinal vesicle is seen at the centre. Fertilization takes place externally in the water medium. Following fertilization, the pyriform eggs assume a spherical shape with the breakdown of the germinal vesicle.
 |
| LIFE CYCLE OF PEARL OYSTER |
LARVAE AND SPAT HANDLING
Larval rearing conditions
Larval density plays a significant role in the growth of pearl oyster larvae. Under identical conditions the larvae show differential growth rate at different larval densities. At higher densities the growth and spatfall are poor. A culture density of two larvae per ml produces optimum growth and spatfall rates. The colour of the culture tanks also influences the setting of larvae. Spatfall is much higher in FRP black tanks than white and blue tanks. Aeration during larval rearing affects growth and spatfall. The effect of aeration is more pronounced in smaller volumes of water. However, aeration is required after the setting of the pearl oyster larvae.
Spat production
Spat production is carried out in the molluscan hatchery at Tuticorin throughout the entire year. However, during May-August the spatfall is less due to high salinity, dustfall and warm landward wind. Sudden spurt of ciliates in the culture medium is common during this period. Such problems can be overcome by good management.
Feeding
The microalgal cell Isochrysis galbana is provided to the larva from the veliger stage onwards. The optimum ration for a larvae is 5,000 cells/day up to umbo stage. The dose is doubled from the umbo to the pediveliger stage and tripled afterwards up to settlement. For about 15 days after settlement each spat is fed with I. galbana at 50,000 cells/day. Mixed algal diet containing mostly Chaetoceros and I. galbana is given in a ratio of 1:1 in the following 15 days. Later the spat is supplied with a mixed algal diet.
Transplantation
The spat are reared in the hatchery for about two months. By then they shall have grown to 3 mm or more. They are then transferred to the farm in velon screen netcages with a mesh size of 400 μm. Mortality may occur if spat measuring less than 3 mm are transplanted. Spat growth is monitored carefully and the netcages are changed whenever necessary. The size of mesh of the rearing cages is also monitored. The oyster spat attain an average size of 40–45 mm in 12 months.
PEARL OYSTER FARMING
SELECTION OF CULTURE SITES
Sheltered bays are ideal locations for pearl oyster farms. They offer good protection to the culture structures such as rafts and cages. Shallow coastal waters where the sea is calm most of the year can also be considered as a suitable site.
Environmental conditions
Temperature
In temperate regions, the water temperature plays an important role in the biological activities of pearl oysters. In Japan, the optimum temperature for oyster growth has been found to be between 20–25 °C.A temperature below 13 °C causes hybernation. Below 6 °C, the oysters die. At temperatures above 28 °C, the oysters show exhaustion. The thickness of the pearl layers are affected by the minute changes in water temperature during the day and also vary considerably according to the season of the year.In the Gulf of Kutch, the oysters grow vigorously in winter months when the seawater temperature ranges between 23–27 °C. A slight decrease in temperature triggers spawning in oysters in the Gulf of Mannar.
Salinity
Pearl oysters tolerate a wide range of salinity from 24–50ppt for a short duration of 2–3 days. The effect of salinity on the growth of pearl oyster has not been clearly investigated. However, it appears that pearl oysters tend to prefer high salinities. Oysters raised in such salinities produce pearls with a golden tint.
Bottom
Gravelly bottoms are suitable for pearl oyster farming, while sandy or muddy bottoms should be avoided. Oyster growth is affected by water temperature and nutritional condition of the ground. Repeated culture on the same ground leads to some extent the deterioration of pearl quality.
The chemical and physical state of the sea bottom is affected by the organic substances discharged from the oysters and fouling organisms. Periodic removal of such accumulated substances from the bottom of the culture grounds often increase production as well as quality.
Depth
The optimum depth for farming pearl oysters is around 15 m. At greater depths, even if the rate of nacre deposition is slower, pearls of high quality with a pinkish colouration are obtained.
Silt load
Pearl oysters generally prefer clear waters as high turbidity levels will affect their filtration efficiency.
Water current
In strong water currents the formation of the pearl layers is usually fast, but the quality of pearls produced is affected.
Primary productivity
The condition of a specific culture ground depends primarily on the chemical constitution of the seawater and on the species and amount of plankton present. Rich nutrients discharged by rivers into the sea are responsible for high primary productivity. The oysters probably derive their chief source of conchiolin from the nitrogen substance of the plankton.The organic matter and calcium dissolved in the seawater are directly absorbed by the food consumption cells. The calcium passes through the mantle to be deposited on the surface of the shell or pearl in the process of their formation. The presence of trace metals in small quantities influences the colour of the nacre.
SUPPLY OF PEARL OYSTERS
In pearl oyster farming, oysters collected from the natural beds or reared from naturally collected or cultured spat are used. In the Gulf of Mannar, several pearl banks are distributed off Tuticorn at a distance of 12–15 km and at depths of 12–25 m. Pearl oysters from these beds are collected by skin and SCUBA diving.In the Gulf of Kutch, the pearl oysters are found on the intertidal flats and the population is sparse. Collection is done by hand.In Japan, oyster spat are collected by submerging bundles of cedar twigs near the water surface during the peak larval settlement season. Hyzez films and old fish nets are also commonly used as spat collectors. Spat collection attempts in India have not been successful, and this may be due to the distance of the pearl oyster beds from coastal waters. However, India has recently succeeded in producing pearl oyster seed under hatchery conditions, therby providing the industry with a more dependable source of oysters.
REARING METHODS
Raft culture
Raft culture is considered to be one of the most suitable farming methods in sheltered bays.A raft of 6×5 m in size can be easily constructed and floated with 4 buoys.
Rafts are usually constructed with logs of teak, venteak or casuarina wood, of chosen length with the bottom of about 10 cm diameter tapering to 6 cm diameter at the tip. These logs are arranged as per the requirement and lashed with coir ropes. Floats are attached to the raft to give buoyancy. The floats can be sealed empty diesel drums of 200 l capacity with fibreglass coating, mild steel barrels painted with antisaline/anticorrosive paints or FRP styrofoam floats. Rafts are moored with anchors at opposite sides with tested quality chains and their direction is decided according to the prevalent wind direction at the specific site.In the long-line culture method, spherical or cylindrical floats which are connected by horizontal synthetic rope or chain are used. The oyster cages are suspended from the ropes.
This system is good for open sea conditions. In another method of hanging, a hole is drilled near the hinge of the pearl oyster. A small thread is put through the hole, which is then tied to a straw rope coated with tar. The straw ropes are hung from a raft.
On-bottom culture
Sea bottoms with a granite or coral stones composition can be used for on-bottom culture.
In the Tuticorin Harbour Basin where the breakwater has been constructed with granite stones, the protected portion of the breakwater is used for culturing mother oysters.
1 m of water is available below the low water mark. Due to constant circulation of seawater, settlement of fouling organisms is poor and inconsistent.
However, it has been noted that the growth of the mother oyster is slower in on-bottom culture compared to the growth of oysters cultured in raft.
Rearing containers
Culture of mother oysters
Box cages, measuring 40×40×15 cm, are used to rear mother pearl oysters.
The size of the mesh varies with the size of the oysters to be reared. The frames of the cages are made up of 6 mm mild steel rods, coated with anticorrosive paints or coal tar. Box-cages are useful in general mother oyster culture. To trace the history and performance of individual oysters, frame nets are used. The frames, measuring 60×40 cm each with five compartments, meshed and hinged at one end, open as a book. The oysters are arranged in rows and held in the compartments when closed. The space available in between the two frames is about 10 mm which is sufficient for the oysters to open their valves for feeding and respiration.
 | ||
| (A) Culture raft constructed with teak poles; (B) A FRP styrofoam buoy; (C) A mild steel buoy (D) Oyster long-line culture system. |
Juvenile rearing
Juvenile pearl oysters are reared in netcages . Synthetic fabric of velon screen bags whose sides are stretched with a steel rod in the form of a prism are used for rearing of juveniles. The mesh size of the screen depends on the size of juveniles to be reared. The mouth of the bag is tied with a synthetic twine which facilitates opening or closing when required. To provide further protection from predators the bags are placed in old nylon fish net bags. Clogging by silt and by the growth of fouling organisms can be prevented by periodical replacement of the velon screen bag which can be cleaned, sun-dried and reused. Spat of up to 2 cm in size are reared in these small netcages. Box-cages which are used for rearing mother oysters can also be used for juvenile rearing by providing an additional velon screen cover inside the cage.
PEARL FORMATION
Natural pearl formation
The principal causative factor in pearl formation in a pearl oyster is the presence of a nucleus.
It can be of organic or inorganic origin, such as parasites adults or larvae, molluscan eggs, decaying parts of plants, sand grains, epithelium or blood cells of the same animal, etc..
These tiny particles or organisms enter the oyster when the shell valves are open for feeding and respiration. These foreign bodies may become embedded between the shell and mantle.
In response to this stimulus, the foreign body is invaginated by the outer epithelium of the mantle and a pearl-sac is formed around it. Pearls are not produced without the formation of the pearl-sac.
The pearl-sac is derived from the internal or external layer of the apithelium of the mantle or of the gill plates. The epithelial cells of the pearl-sac secrets the nacre which becomes deposited over the foreign body, forming a pearl in due course of time. These pearls are produced either within the mantle, in other soft tissues of the oyster, or between the mantle, and the interior surface of the shell.
Such pearl production is accidental and occurs very rarely. They are generally small and irregular. Large and spherical pearls are still rarer to find. When the extraneous matter becomes fixed to the shell, only the exposed portion becomes covered by the pearl-sac resulting in a blister pearl.
Cultured pearl formation
Cultured pearls are formed in a pearl oyster, thanks to human interference.
In any pearl formation, two things are required, the outer epithelium of the mantle lobe and core substance or nucleus. It was found that cut pieces of the mantle epithelium would provide the pearl secreting cells and that processed shell beads would be accepted by the oyster as the foreign body.
Through careful surgery, the mantle piece graft tissue and the shell bead nucleus are implanted together, side by side, into the gonad of the oyster.The oysters are then returned to sea for further growth. The outer epithelial cells of the graft tissue proliferate and rearrange themselves over the shell bead nucleus, forming a pearl-sac. The inner epithelium and connective tissue of the mantle disintegrate and become absorbed by the surrounding tissue. The cells of the pearl-sac derive their nourishment from the surrounding tissues and soon reassume their function of nacre (mother-of-pearl) secretion which is deposited over the nucleus in the form of concentric micro-layers. The nacreous matter consists of thin alternate layers of aragonite and conchiolin deposited around the nucleus. The conchiolin is organic in nature and consists of mucopolysaccarides. It forms the binding layer for the aragonite crystals.The aragonite layers are 0.29–0.60 mm thick and are made of calcium carbonate in the form of highly laminated crystals. In cultured pearls the nacre quality and the process of pearl formation are the same as in the formation of natural pearls. Cultured half-pearls are produced by affixing many nuclei on the inner surface of the shell valves. The outer epithelium of the mantle forms the pearl-sac on the free surface of the nucleus and the halfpearl is formed.
QUALITY OF PEARL
To achieve a high rate of production of quality pearls, the following factors are required to be taken care of:
OYSTER SELECTION
Large oysters, in terms of size and weight should be selected.
They must be free from a heavy fouling load and blisters caused by sponges and polychaetes.
The oysters should be healthy as can be judged from the colour of the visceral mass and gills.
GRAFT TISSUE PREPARATION
The graft tissue is one of the most critical factors in controlling the rate of pearl production.
The donor oyster should be of the desirable size with a well developed and healthy mantle. Extreme care should be taken in selecting, stretching, cleaning, trimming and cutting of the donor mantle tissue. Good water quality and correct level of the chemical agents should be used in maintaining the tissue pieces.
IMPLEMENTATION
The nucleus implantation is one of the most important factors in cultured pearl production.
Its success greatly depends on the selection of the correct site and skill of the technician.
The positioning and orientation of graft tissue in contact with nucleus is also critical and should be carried out with great skill and patience.
Multiple nucleus implantation requires still greater care and patience.
OYSTERS CONVALESCENCE
Oysters can be made to recover from the effect of narcotization through periodic changes of water or gentle flow-through. Sufficient time must be allowed for the incision wound to heal before taking the oysters to the sea for further farming.
TOOL MAITENANCE
The tools must be sharp, rust-free and should have been either sterilized or suitably cleaned and sun-dried.
COLOUR OF PEARL
Different molluscs produce pearls of different colours. This is very clearly shown by Pinctada margaritifera (black or steel grey), P. maxima (silvery white), abalones (green) and freshwater mussels (pink). However, in the case of P. fucata, the colour of the pearls produced may be golden yellow, pink, white or cream, depending on slight differences in the site of nuclei implantation.
A number of environmental factors plays a predominant role in determining the colour and lustre of the pearl nacre. Water depth is one of the most important factors, as quality pearls tend to be produced in waters below 10 m. Fouling and boring problems and siltation are considerably less at depths of 10 m or more.